
New Drug Designations - July 2024
Shots:
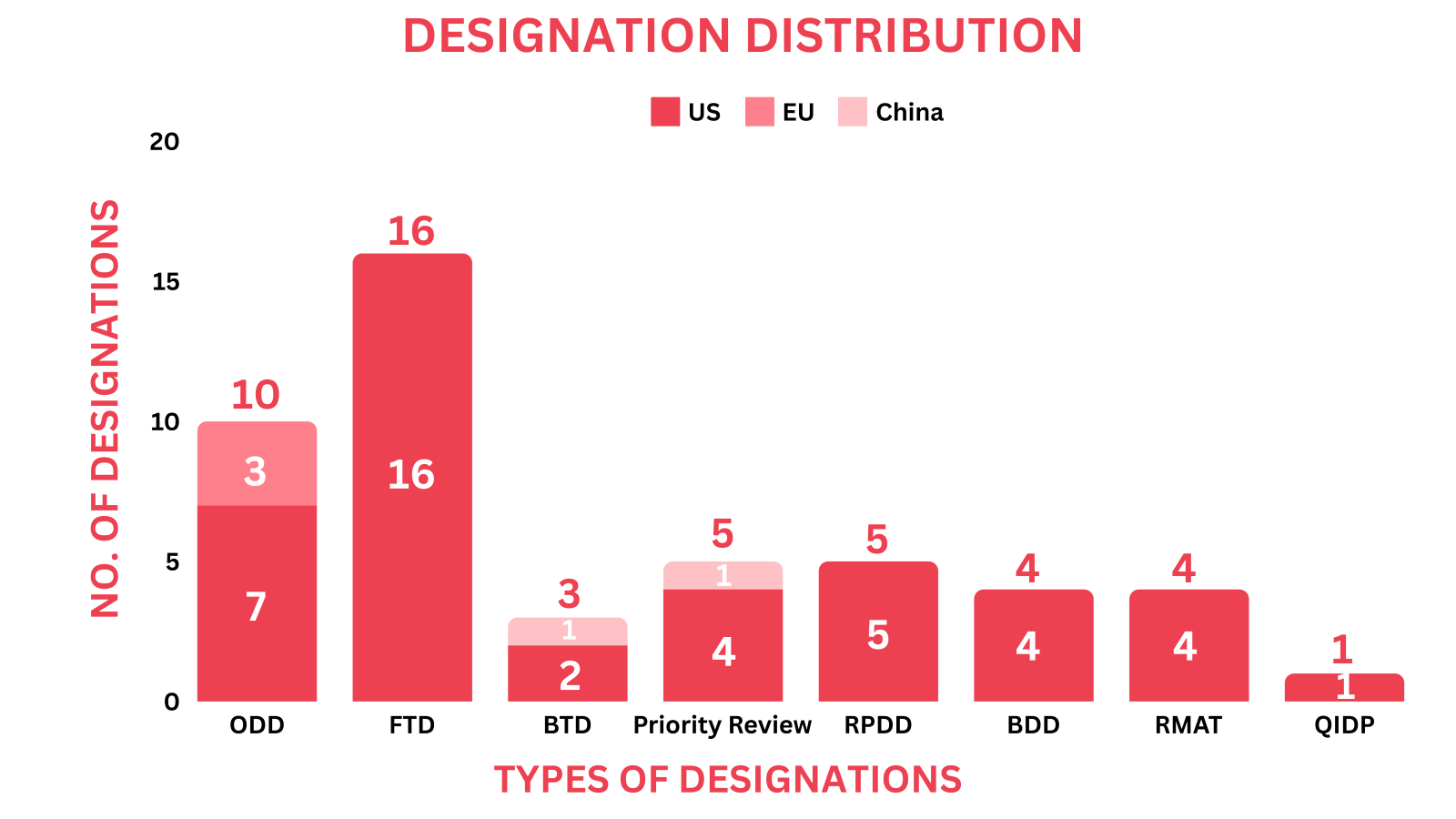
- PharmaShots' designation report provides a concise overview of several drugs and their designations by the FDA, NMPA and EMA. This month’s report includes designations allotted to 16 small molecules, 5 biologics, 11 cell & gene therapies, 1 recombinant protein, 1 vaccine, 1 immunotherapy, 1 radiopharmaceutical, 1 antisense oligonucleotide, 3 RNA therapies 1 herbal medicine and 5 devices
- This month, Cellectis’ UCART22 received the US FDA’s ODD & RPDD for Acute Lymphoblastic Leukemia
- We have compiled a list of a total of 39 drugs and 5 devices awarded with designations by multiple regulatory bodies in July 2024

UCART22
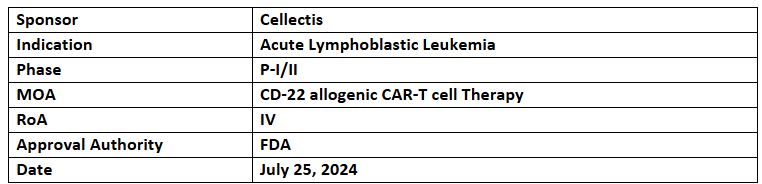
- It has also received the US FDA’s RPDD for ALL
- The P-I/II (BALLI-01) dose-escalation & expansion study assesses the safety, expansion, persistence & clinical activity of UCART22 (allogeneic CAR T-cell therapy targeting CD22) to treat r/r ALL
- The results, highlighted at ASH 2023, showed a 67% response rate at dose level 2 vs 50% response rate at dose level 3 with UCART22-P1. Further updates are expected by YE’24
Avutometinib and Defactinib
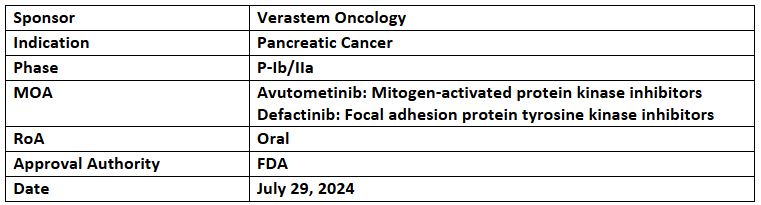
- The US FDA has granted ODD to avutometinib + defactinib for treating pancreatic cancer. A rolling NDA submission has been initiated for the combination in recurrent LGSOC, to be completed in H2’24, with the US FDA’s decision anticipated in H1’25
- Avutometinib is being assessed under the P-Ib/IIa (RAMP 205) trial for its safety, tolerability & efficacy combined with defactinib & SoC CT (gemcitabine & nab-paclitaxel) in treatment naïve pancreatic cancer patients, with part A finding RP2D for proceeding to part B. 41 patients were treated in 4 dose cohorts
- Interim data (as of May 2024), highlighted at ASCO 2024, showed confirmed PR in 83% of patients at >6mos. follow-up & a single DLT that was cleared after additional patient recruitment in dose level 1; 21/26 patients in all cohorts who had their first scan, experienced a reduced target lesion sum of diameters. Further data anticipated in Q1'25
7MW3711
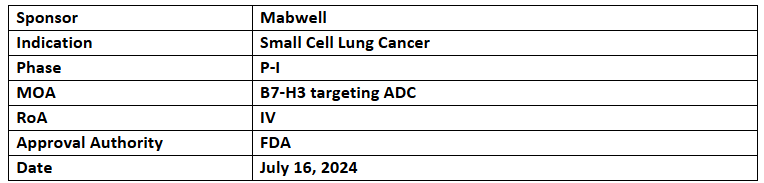
- 7MW3711 consists of an antibody molecule, novel linker & novel payload Mtoxin (TOP1i), developed using IDDC platform & targets B7-H3. It works by binding with tumor cells to release Mtoxin in the lysosome, inducing tumor cell apoptosis
- 7MW3711 showed tumor killing in animal models plus a good safety profile in cynomolgus monkeys, with controlled on-target and off-target toxicities
ZMA001
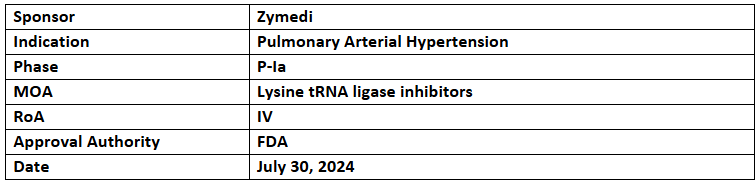
- ZMA001 is being evaluated under P-Ia study for its safety & tolerability among healthy adults to identify dosing and side effects at NIH. It began in Jan 2024
- Non-clinical studies showed the superior effectiveness of ZMA001. It was also effective in combination with existing therapies
- ZMA001, a human mAb, blocks inflammation-inducing macrophages from entering the lungs, inhibiting PAH symptoms from the early stages
PSX-514
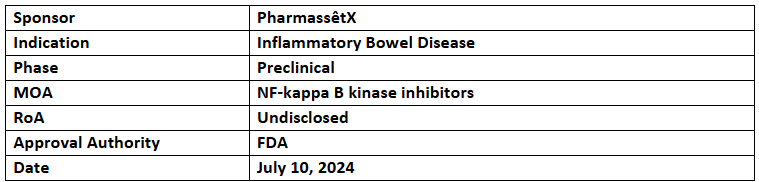
- The designation includes pouchitis which is a rare form of IBD
- Peer-reviewed evidence published in the Inflammatory Bowel Diseases showed that an EGCG-enriched green tea extracts improved symptoms (incl. endoscopic improvement) in patients with mild to moderate UC, without any SAEs
- A retrospective study published in the International Journal of Colorectal Diseases found that most refractory pouchitis patients experienced complete symptom relief with EGCG, without any SAEs
- PharmassêtX partnered with Alimentiv to accelerate patient access for the development of PSX-514, aiming to advance it into the trials in 2025
IN022
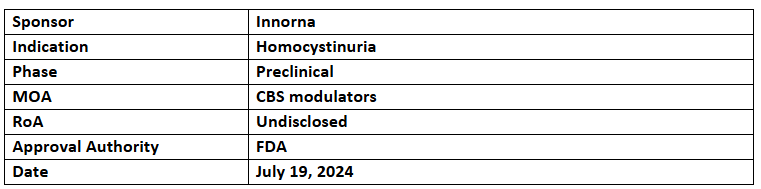
- IN022 has received ODD for treating HCU
- IN022, an mRNA therapy developed using mRNA-LNP technology, works by restoring CBS enzyme function to normalize homocysteine metabolism, improving symptoms in HCU patients
IN016
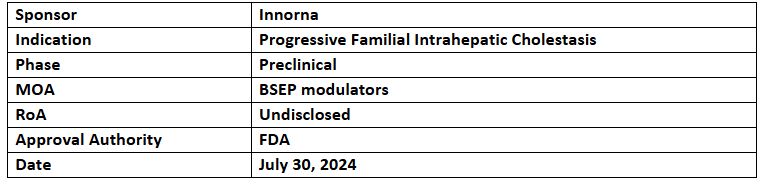
- IN016 has received ODD for treating PFIC
- IN016 aims to restore defective proteins to normalize bile excretion for improving symptoms of PFIC
SLS009
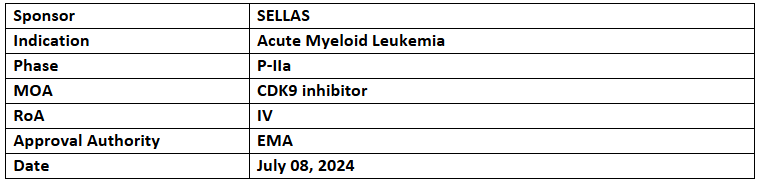
- The P-IIa study assesses the safety, efficacy & tolerability of SLS009 [45mg & 60mg (QW or 30mg, BIW)] + aza/ven for treating AML
- Data readout from the P-IIa study is expected in Q3 2024
- Patient recruitment continues across the 2 arms, one with ASXL1 mutations and the other with myelodysplasia-related mutations excl. ASXL1
FT011
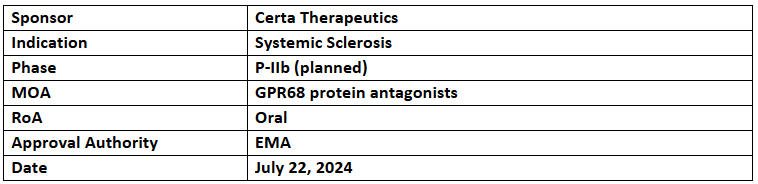
- The EMA has granted orphan drug designation to FT011 for treating Systemic Sclerosis (SSc). It has been designated with the US FDA’s ODD & FTD for the same
- FT011 depicted favorable effectiveness across in vitro & in vivo inflammatory and fibrotic disease models with its P-I & P-IIa showing favorable efficacy, safety & PK. Certa plans a P-IIb confirmatory study and is developing biomarkers & gene signatures for identifying patients, likely to respond to fibrosis treatment
- FT011 (oral) is an FIC therapy that works by targeting GPR68, a membrane GPCR receptor, responsible for fibrosis
KIO-301
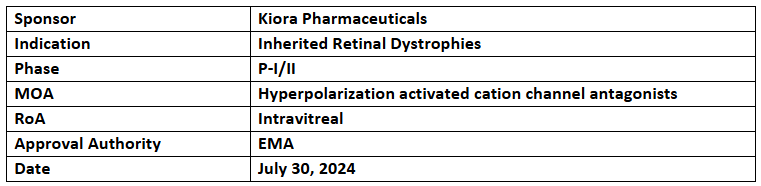
- The designation of KIO-301 includes non-syndromic rod-dominant retinal dystrophies
- KIO-301 will be investigated under ABACUS-2 study to restore vision among RP subjects
- Kiora & Théa Open Innovation (TOI) entered into an exclusive global co-development & commercialization agreement (excl. Asia) for KIO-301 to treat retinal diseases in Jan 2024
- KIO-301 is a small molecule photo-switch that restores light-sensing abilities to retinal ganglion cells after photoreceptor degeneration in inherited retinal diseases such as retinitis pigmentosa

9MW2821
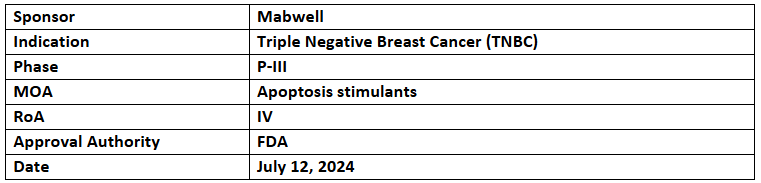
- Mabwell’s 9MW2821, nectin-4-targeting ADC, has received the US FDA’s FTD to treat locally advanced or metastatic Nectin-4 +ve TNBC
- In last 6mos., 9MW2821 received multiple designations from the FDA received
- FTD for the treatment of advanced, recurrent, or metastatic esophageal squamous cell carcinoma (ESCC) and recurrent or metastatic cervical cancer (CC) progressed on or following prior treatment with a platinum-based chemotherapy regimen, and
- Granted ODD for the treatment of esophageal cancer (EC), highlighting its potential and innovation in treating multiple tumors.
Diamyd
- The US FDA has granted FTD to treat pediatric patients with type 1 diabetes (stage 1 or 2) with HLA DR3-DQ2 genotype
- It also received FTD for stage 3 type 1 diabetes in subjects with HLA DR3-DQ2 genotype in early 2024
Tonmya (TNX-102 SL)
- Tonmya received the US FDA’s FTD for the management of fibromyalgia; decision supports to market drug in 2025
- In Dec 2023, the company reported topline data from the P-III (RESILIENT) trial of Tonmya for fibromyalgia that reached the 1EP, showing reduced daily pain vs PBO. It also showed significant improvements in sleep quality, fatigue, and overall fibromyalgia symptoms across all 6 2EPs
- Tonix confirmed alignment with the FDA on its proposed NDA submission for Tonmya in H2’24
- Tonmya is a non-opioid drug intended for treating fibromyalgia, a chronic pain condition mainly affecting women
ADI-270
- The US FDA has granted FTD to treat metastatic/advanced clear cell renal cell carcinoma (ccRCC) patients previously treated with an immune checkpoint inhibitor and a VEGF inhibitor.
- ADI-270 is an armored 3rd gen allogeneic “off-the-shelf” gamma delta CAR T cell therapy for the treatment of CD70-positive cancers.
AB-1005
- Bayer & AskBio (Bayer’s subsidiary) has received the US FDA’s Fast Track Designation along with UK MHRA’s Innovation Passport for treating Parkinson’s disease
- The 18mos. P-Ib study of AB-1005 (AAV2-GDNF gene therapy) for Parkinson’s disease reached its primary safety objective for a one-time bilateral delivery to the putamen
- Moreover, the company is recruiting patients for P-I study evaluating the preliminary safety, tolerability, and efficacy of AB-1005 for parkinsonian subtype of multiple system atrophy (MSA-P) across the US and for P-II (REGENERATE-PD) study across the US with its extension to EU & UK planned in H2’24
RABI-767
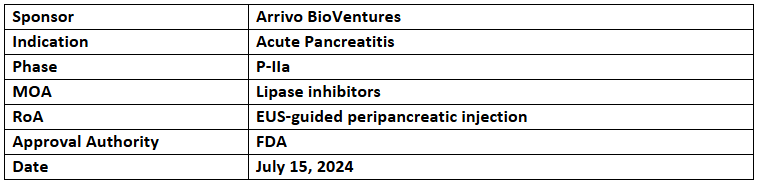
- Panafina (Arrivo’s subsidiary) has reported the US FDA’s fast track designation granted to RABI-767 for treating severe acute pancreatitis
- The P-IIa study is investigating the safety & efficacy of a single dose of RABI-767 + SoC (delivered using endoscopic ultrasound-guided peripancreatic injection) vs SoC alone among patients at risk for severe acute pancreatitis
- RABI-767, a small molecule lipase inhibitor, stops the highly toxic cascade of fat necrosis that is responsible for tissue injury, systemic toxicity, organ failure and mortality in severe acute pancreatitis
DSP-5336
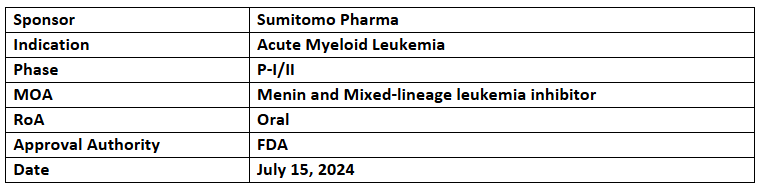
- The FTD has been granted to DSP-5336 for treating r/r AML with KMT2A rearrangement or nucleophosmin mutation (NPM1m)
- DSP-5336 is being assessed under P-I/II dose escalation & expansion study for its safety & efficacy to treat r/r AML
- Updated data was highlighted at EHA 2024, demonstrating 57% ORR in both NPM1 mutation and KMT2A rearrangement with a 24% complete remission or complete remission with partial hematologic recovery (CR/CRh) rate. This builds on preliminary data from ASH 2023
- DSP-5336 works by targeting the menin-MLL protein interaction, crucial for gene expression, cell growth, genomic stability, and hematopoiesis
Lomecel-B

- Lomecel-B, an allogeneic cell therapy, is under investigation for various conditions such as Alzheimer’s disease (P-IIa completed), Aging-related Frailty (P-IIb completed) & HLHS (P-IIb underway)
- Longeveron shared top-line data from P-IIa (CLEAR MIND) study in Oct 2023, with additional data in Dec 2023. Complete results will be presented at the AAIC 2024 on Jul 28, 2024
Ozuriftamab Vedotin (CAB-ROR2-ADC)
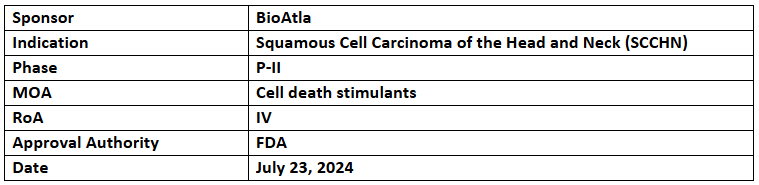
- The FTD has been granted for treating recurrent or metastatic SCCHN progressed on or post Pt-based CT and anti-PD-1/L1 antibody therapy
- Its P-II trial has demonstrated positive results in treatment-refractory SCCHN patients with a median of 3 prior therapies & a manageable safety profile without any new concerns. Discussions with the FDA for a potential registrational trial are planned in H2’24
- Ozuriftamab vedotin is an ADC targeting ROR2, a receptor tyrosine kinase found in various solid tumors such as head and neck, lung, TNBC, and melanoma
Foralumab
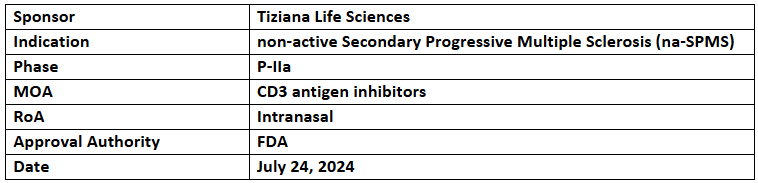
- 10 patients with na-SPMS have shown improvement or disease stability within 6 months in an EAP. The FDA has approved enrolling 20 more patients. Additionally, foralumab is being studied in a P-IIa trial for na-SPMS
- Foralumab is developed to regulate the immune system and reduce neuroinflammation in neurodegenerative diseases like MS, Alzheimer’s, and ALS. Initial data depicted its therapeutic benefits with a favorable safety profile
ACI-35.030 (now JNJ-2056)
- The P-IIb (ReTain) study assesses JNJ-2056 vs PBO in patients (n=~500) with Alzheimer’s disease for 4yrs. It aims to evaluate if it can delay or prevent cognitive impairment by inhibiting Tau pathology. The trial is currently recruiting participants
- JNJ-2056 is developed under a global license agreement with Janssen, and the ReTain trial is funded & conducted by Janssen
IMM-1-104
- The FTD has been granted to IMM-1-104 as a 1L treatment of PDAC. In Feb 2024, it gained FTD as a 2L treatment of PDAC in those who did not respond to 1L treatment
- The patient recruitment for 1L pancreatic cancer is underway in P-IIa trial, which has two arms assessing IMM-1-104 with CT. Trial also includes monotx. arms for 1L & 2L pancreatic cancer, RAS mutant melanoma & RAS mutant NSCLC. Initial data from multiple study arms will be shared in H2’24
SYNC-T SV-102
- Interim P-I data for SV-102 in mCRPC showed 85% ORR and a favorable safety profile. The designation follows IND clearance, with US studies anticipated in H2’24
- SYNC-T SV-102 consists of an in-situ vaccine & intratumoral SV-102 infusion. It aims to stimulate the immune system and block immune suppression, activating T cells for a systemic anti-tumor response
225Ac-FL-020
- The US FDA has granted fast track designation to 225Ac-FL-020 for treating metastatic castration-resistant prostate cancer (mCRPC). Its IND for P-I was approved in May 2024 by the US FDA
- The P-I trial is intended to investigate the safety, tolerability and anti-tumor activity of 225Ac-FL-020 (a next-gen. UniRDC platform-based PSMA-targeting radionuclide drug conjugate) for the treatment of mCRPC
- 225Ac-FL-020 showed anti-tumor activity and a favorable safety profile in LNCaP xenograft mice. However, radiolabeled FL-020 exhibited a favorable in vivo biodistribution profile, consistent tumor uptake & a fast systemic clearance in non-clinical studies
OBX-115

- The US FDA has granted fast track designation to the company’s OBX-115 for metastatic or locally advanced melanoma patients who have relapsed and refractory after treatment with PD-1/PD-L1–based immune checkpoint inhibitors (ICI)
- OBX-115 is currently being assessed in a study for advanced or metastatic melanoma and non-small cell lung cancer (NSCLC), with recruitment completed in another FIH trial
- OBX-115 is an engineered TIL cell therapy featuring pharmacologically regulatable membrane-bound IL-15
HP518
- The US FDA has granted FTD to the company’s HP518 (oral PROTAC AR degrader that targets both wild-type AR & AR LBD mutants such as L702H for treating AR+ triple-negative breast cancer (TNBC)
- HP518, in AR+ TNBC animal models, depicted favorable anti-tumor activity and a good safety profile. The company will provide updates on existing IND (IND 164902) for TNBC in the future
- In addition, HP518 is also being investigated for mCRPC under a P-I/II study across China with its P-I study across Australia showing various long-term PSA50 & partial responses plus favorable safety

Cytisinicline

- The BTD has been granted for treating nicotine e-cigarette, or vaping, cessation
- The P-II (ORCA-V1) study assessed cytisinicline (3mg) vs PBO, TID for 12wks., plus standard behavioral support in 160 daily e-cigarette users across the US. The trial, supported by $2.8M funding from NIDA, used the same cytisinicline dose as in P-III smoking cessation trials
- Results, published in the JAMA Internal Medicine, showed that cytisinicline-treated participants were 2.6 times more likely to quit vaping. It also demonstrated consistent benefit in 2EPs & a well-tolerated safety, without SAEs & high compliance
Bexicaserin (LP352)
- The BTD has been granted for the treatment of seizures associated with Developmental and Epileptic Encephalopathies (DEEs) patients (age ≥2yrs.)
- The FDA will guide the development of bexicaserin to begin its P-III trial in H2’24
AHB-137
- AusperBio’s AHB-137 has received the NMPA’s breakthrough therapy designation for the treatment of chronic hepatitis B (CHB) infection, expediting its development
- The designation was supported by P-I/IIa (China) and P-I (international) studies evaluating the safety & efficacy of AHB-137 for treating CHB infection. Data was highlighted at EASL 2024
- AHB-137 is an unconjugated antisense oligonucleotide (ASO) based on AusperBio's Med-Oligo ASO technology to cure chronic hepatitis B, with its non-clinical data highlighted at the EASL 2023

Crinecerfont
- The US FDA has accepted & granted priority review to the NDA of crinecerfont to treat classic congenital adrenal hyperplasia (CAH) in children, adolescents & adults
- The NDAs contain the effectiveness and safety data of crinecerfont as a capsule formulation (PDUFA: Dec 29, 2024) & as an oral solution formulation (PDUFA: Dec 30, 2024)
- The P-III (CAHtalyst) trials assessed the safety, efficacy & tolerability of crinecerfont in patients with CAH due to 21-hydroxylase deficiency. Results for both pediatric and adult patients were published in The New England Journal of Medicine on Jun 2 and Jun 1, 2024, respectively; also highlighted at ENDO 2024
Vanzacaftor/Tezacaftor/Deutivacaftor
- The US FDA has accepted Vertex’s NDA for the vanzacaftor/ tezacaftor/ deutivacaftor triple therapy (QD) for cystic fibrosis patients (≥6yrs.) with at least one F508del mutation or another responsive mutation in the CFTR gene
- It utilized a priority review voucher and received the PDUFA date of Jan 2, 2025
- The MAA for the same has been validated by the EMA, with further submissions made in Canada, Australia, Switzerland & the UK
Tabelecleucel
- The US FDA has accepted & granted priority review (PDUFA: Jan 15, 2025) to the BLA of tabelecleucel (tab-cel) alone to treat adults & pediatrics (≥2yrs.) with EBV+ post-transplant lymphoproliferative disease. Pierre Fabre & Atara anticipate the US launch in early 2025
- The BLA was based on the data from studies involving >430 patients, incl. ALLELE trial, showing ORR of 48.8% with a favorable & consistent safety profile
- Atara will get a $20M milestone for BLA acceptance from Pierre Fabre with another $60M on FDA approval plus sales milestones & double-digit tiered royalties across the US & international markets. Pierre Fabre will reimburse the global development costs via the BLA transfer & purchasing tab-cel inventory through the manufacturing transfer date
Scemblix

- The US FDA has granted priority review to Scemblix for treating Philadelphia chromosome +ve CML in chronic phase (Ph+ CML-CP)
- The designation was supported by the P-III (ASC4FIRST) trial assessing the efficacy, tolerability and safety of Scemblix (80mg, QD) vs investigator-selected (IS) SoC TKIs (imatinib, nilotinib, dasatinib & bosutinib) among newly diagnosed adults (n=405) with Ph+ CML-CP
- Scemblix showed MMR rates of 68% vs 49% (SoC TKIs) and 69% vs 40% (imatinib alone) at wk.48. It also had fewer G≥3 SAEs (38% vs 44% & 55%), dose adjustments (30% vs 39% & 53%) & discontinuations (5% vs 11% & 10%). The safety profile was consistent with prior studies, with no new signals
Tazemetostat
- The NMPA has accepted and granted priority review to the NDA of tazemetostat for treating adults with r/r follicular lymphoma
- The NDA was based on P-II bridging trial evaluating safety and PK of tazemetostat for the treatment of r/r FL patients (n=42) with/without EZH2 mutations, with the 1EP as ORR and 2EPs as DoR, PFS & OS across China. Data will be highlighted at future conferences
- Tazemetostat is a FIC EZH2 inhibitor which is developed by Epizyme (an Ipsen company) with HUTCHMED developing, manufacturing & commercializing it across China, Hong Kong, Macau & Taiwan as per an agreement b/w them. It has been approved in the US FDA under accelerated approval for r/r FL and advanced epithelioid sarcoma

UCART22
- It has also received the US FDA’s ODD for ALL
- The P-I/II (BALLI-01) dose-escalation & expansion study assesses the safety, expansion, persistence & clinical activity of UCART22 (allogeneic CAR T-cell therapy targeting CD22) to treat r/r ALL
- The results, highlighted at ASH 2023, showed a 67% response rate at dose level 2 vs 50% response rate at dose level 3 with UCART22-P1. Further updates are expected by YE’24
VCN-01

- It has previously received ODD for retinoblastoma
- The company is planning to advance VCN-01 as an adjunct to CT for retinoblastoma in pediatrics. The results from its investigator sponsored P-I study in pediatric patients with refractory retinoblastoma were +ve
- VCN-01 is an oncolytic adenovirus that replicates in tumor cells while degrading the tumor stroma to enhance cancer treatment
IN022
- IN022 has received RPDD for treating HCU
- IN022, an mRNA therapy developed using mRNA-LNP technology, works by restoring CBS enzyme function to normalize homocysteine metabolism, improving symptoms in HCU patients
IN016
- IN016 has received RPDD for treating PFIC
- IN016 aims to restore defective proteins to normalize bile excretion for improving symptoms of PFIC
RAG-18
- RAG-18 is an saRNA candidate that targets and activates UTRN gene expression in muscle cells using RNAa mechanism

Nanosecond Pulsed Field Ablation (nsPFA) technology
- The BDD has been granted for cardiac tissue ablation to treat AF
- Pre-clinical studies have shown a consistent, transmural ablation with a single application of the system for <2sec. Nano-PFA’s non-thermal mechanism prevents thermal spread, reducing the risk of collateral tissue injury compared to thermal radiofrequency ablation
- The company anticipates PMA application for its commercialization across the US to treat AF. It is also planning to initiate pivotal AF study in 2025, with further details in H2’24
- The non-clinical results were favorable and similar outcomes are expected in upcoming clinical procedures across the Netherlands in 2024
- The system uses a bipolar clamp with nanosecond PFA technology to create durable, continuous ablation lesions for treating atrial fibrillation
ContraBand System
- The US FDA has granted BDD to the company’s ContraBand System for treating heart failure with reduced ejection fraction (HFrEF) in patients, symptomatic to optimal medical therapy, without significant pulmonary hypertension or right heart failure
- The designation was supported by an ongoing feasibility trial that showed a reduction in left ventricular volume with improvements in hemodynamic function & physical capacity
- ContraBand device is a minimally invasive transcatheter Pulmonary Artery Banding (PAB) system intended to improve quality of life in patients with left ventricle failure
neuro Optical Coherence Tomography (nOCT) Technology
-
nOCT is a specialized intravascular imaging technology for cerebrovascular navigation, offering detailed brain vessel visualization at near histologic levels to enhance diagnostics and treatment precision
Revita System
- The BDD has been granted to Revita system to maintain weight loss post GLP-1 drugs discontinuation. BDD will grant priority FDA review after the REMAIN-1 study and may lead to faster reimbursement decisions by CMS
- It’s REMAIN-1 pivotal study is underway, with data expected in Q4’24 & a mid-point randomized analysis in Q2’25
- Previous clinical studies of Revita in T2D patients across the US & EU suggest durable weight maintenance after a single procedure
- Revita is an outpatient endoscopic procedure to resurface the duodenal lining for improving nutrient absorption

Lomecel-B
- Lomecel-B, an allogeneic cell therapy, is under investigation for various conditions such as Alzheimer’s disease (P-IIa completed), Aging-related Frailty (P-IIb completed) & HLHS (P-IIb underway)
- Longeveron shared top-line data from P-IIa (CLEAR MIND) study in Oct 2023, with additional data in Dec 2023. Complete results will be presented at the AAIC 2024 on Jul 28, 2024
KYV-101
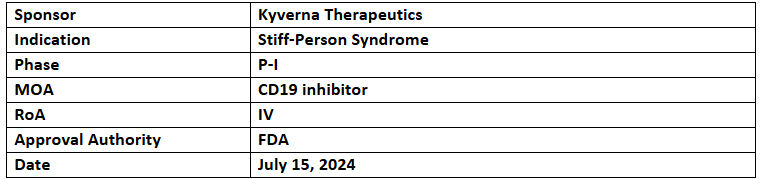
- The designation has been granted to treat refractory stiff-person syndrome
- The application was supported by positive results of KYV-101 in German patients under a named-patient treatment option
- KYV-101 is being evaluated in P-I/II (US) and P-II (Germany) to treat autoimmune diseases in rheumatology and neurology
- KYV-101 is an autologous CD19 CAR T-cell therapy for B cell-driven autoimmune diseases, designed by the NIH. It showed improved tolerability in P-I oncology study (n=20), with data published in Nature Medicine
AIC100
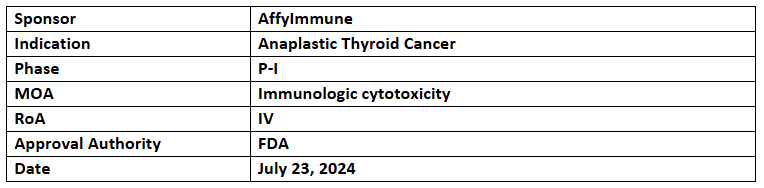
- The US FDA has granted RMAT designation to AIC100 (ICAM-1 targeting & affinity-tuned LFA-1 binder CAR-T cell therapy) for treating recurrent anaplastic thyroid cancer (ATC)
- The designation was based on interim data, highlighted at ASCO 2024, from first 10 patients involved in P-I trial evaluating the safety, tolerability & RP2D of AIC100 to treat r/r poorly differentiated thyroid cancer (PDTC) & ATC
- As of the data cut off, results in 10 patients receiving AIC100 at 3 dose levels showed no DLTs with MTD not attained, DCR of 67% and ORR of 33% in DL2 (100 million CAR T cells) & DL3 (500 million CAR T cells) with 1 durable PR (DL2) & 1 ongoing durable mCR (DL3)
Acellular Tissue Engineered Vessel (ATEV)
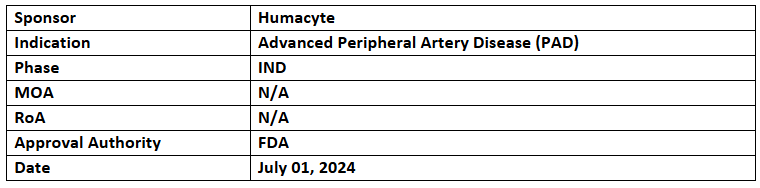
- The RMAT designation was granted, as the FDA cleared an IND application of Acellular Tissue Engineered Vessel (ATEV) for PAD
- The ATEV has been studied in two P-II PAD trials with up to 6yrs. of follow-up; the Mayo Clinic is also evaluating ATEV in chronic limb-threatening ischemia patients (n=30)
- Humacyte’s ATEV is an off-the-shelf implant designed for use in vascular replacement & repair. It showed a low infection rate in studies, offering surgeons a ready-to-use solution that could save time and improve patient outcomes

CSA-131
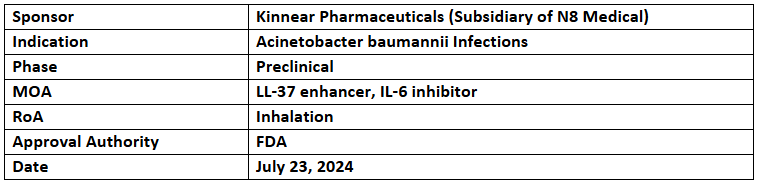
- The QIDP designation has been granted to CSA-131 for preventing Acinetobacter baumannii infections in cystic fibrosis patients. It was previously designated with QIDP for preventing & treating infections caused by Candida auris, Aspergillus, Pseudomonas & Klebsiella pneumoniae pathogens among cystic fibrosis patients
- CSA-131 is an inhaled drug of class Ceragenins, a synthetic non-peptide mimic of the antimicrobial peptide LL-37 in the body's innate immunity against lung infections
References
- Cellectis
- Verastem Oncology
- PRNewswire
- Globenewswire
- Businesswire
- AskBio
- BioAtla
- Tiziana Life Sciences
- AC Immune
- Immuneering Corporation
- Syncromune
- Full-Life Technologies
- Longboard Pharmaceuticals
- Neurocrine Biosciences
- Vertex Pharmaceuticals
- Novartis
- HUTCHMED
- Theriva Biologics
- Innorna 3
- Innorna 4
- Ractigen Therapeutics
- Pulse Biosciences
- Longeveron 2
- Humacyte
- Kinnear Pharmaceuticals
Related Post: New Drug Designations - June 2024
Tags

Disha was a content writer at PharmaShots. She is passionate and curious about recent updates and developments in MedTech and Pharma industry. She covers news related to clinical trial results and updates. She can be contacted at connect@pharmashots.com.














